D-amino acids in biocatalysis
A universal chemical rule states: «Synthesis of chiral compounds from achiral reagents always yields the racemic modification» and «optically inactive reagents yield optically inactive products».
To resolve a racemate another homochiral compound is required: actually, (R) and (S) compounds have identical properties except when they interact with other chiral phenomena. E.g., the products of the reaction of the (R) and (S) enantiomers with an (S’) substance generates R-S’ and S-S’ compounds (named diasteroisomers) which are not mirror images and thus differ in physical properties such as solubility.
Asymmetric autocatalysis (Franck, 1953): the amplification of an enantiomeric excess by means of a chemical reaction in which the product is a chiral compound and one enantiomer catalyzes its own formation while inhibiting the formation of the opposite enantiomer, e.g. Soai reaction (Soai, 1995).
Mechanisms for enzymatic synthesis of D-amino acids
The synthesis of D-amino acids (D-AA) can be performed by different enzymes starting from D,L-AA mixtures, N-acyl-D,L-AA, D,L-hydantoin, D,L-amides, α–keto acids and L-amino acids.
– Inversion of α-C of the corresponding L-amino acid: amino acid racemases (EC 5.1.1.X) catalyze the formation of a racemic mixture from either a free D- or L-AA by equilibrating configuration at the α-carbon, while amino acid epimerases act on compounds possessing an additional asymmetric carbon: a diastereomer or epimer of the substrate is formed rather than its antipode. Both enzymes are frequently referred to as “racemases”. Racemases can be classified into two main groups: PLP-dependent enzymes vs. cofactor independent enzymes.
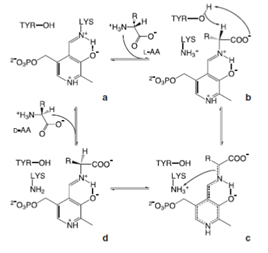
Fig. Mechanism for D-AA synthesis by a PLP-dependent racemase.
– Stereospecific amination: D-amino acid transaminase (D-AAT, EC 2.6.1.21) catalyzes the transfer of the amino group from D-alanine to α–ketoglutarate to yield D-glutammate and pyruvate
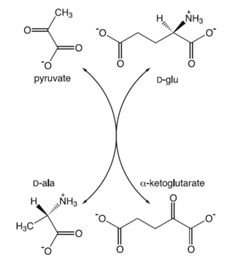
Fig. Mechanism for D-AA synthesis by a PLP-dependent racemase.
Enzymes with a broader substrate acceptance have been also discovered (Kobayashi et al., 2013). The substrate specificity of the α–keto acid determines the D-AA formed (pyruvate is converted into D-alanine, phenylpyruvate into D-phenylalanine, etc.).
– Asymmetric reductive amination of an α–keto acid into the corresponding D-AA by a D-amino acid dehydrogenase (D-AAdH, EC 1.4.99.1). A suitable alternative is represented by meso-diaminopimelate dehydrogenase which shows a wider α–keto acid acceptance (Gao et al., 2012).
– Kinetic resolution of racemic amino acid amides by D-stereospecific amidohydrolase:
- N-acyl-D-amino acid amidohydrolase (EC 3.4.4.14), converting N-acyl-D-AA into the corresponding D-AA and fatty acid (Wakayama et al., 2003).
- D-amino acid amidase (EC 3.5.1.X), which hydrolyzes D-AA amides into D-AAs and ammonia.
- D-aminopeptidase (EC 3.1.11.19), acting on peptides harbouring a D-AA at the N-terminal end.
- D-peptidase (EC 3.4.11.X): the alkaline D-peptidase acts on peptides constituted by aromatic D-AAs.
– Kinetic resolution of racemic amino acid solutions by:
- L-amino acid oxidase (EC 1.4.3.2), catalysing the O2-dependent deamination of L-amino acids into the corresponding α–keto acid, ammonia and hydrogen peroxide, while D-AAs are not converted (Pollegioni et al., 2013).
- L-amino acid deaminase (LAAD, EC 1.4.99.B3), which catalyses the same reaction of LAAO with no O2 consumption and no hydrogen peroxide generation (Molla et al., 2017). The full generation of D-4-nitrophenylalanine from the corresponding L-enantiomer has been obtained by coupling the deamination of the L-amino acid to the achiral imino acid by LAAD to in situ reduction by the borane tert-butylamine (Rosini et al., 2017).
– Multi-step processes:
- D-hydantoinase (EC 3.5.2.2) to hydrolyze D,L-5-substituted hydantoins coupled with the decarbamoylation by a N-carbamoyl-D-amino acid amidohydrolase (EC 3.5.1.77).
References
Frank F.C. On spontaneous asymmetric synthesis. Biochim. Biophys. Acta (1953) 11, 459-463
Gao X., Chen X., Liu W., Feng J., Wu Q., Hua L., Zhu D. A novel meso-diaminopimelate dehydrogenase from Symbiobacterium thermophilum: overexpression, characterization, and potential for D-amino acid synthesis. Appl. Environ. Microbiol. (2012) 78, 8595–8600.
Kobayashi J., Shimizu Y., Mutaguchi Y., Doi K., Ohshima T. Characterization of D-amino acid aminotransferase from Lactobacillus salivarius. J. Mol. Catal. B Enzym. (2013) 94, 15–22.
Molla G., Melis R., Pollegioni L. Breaking the mirror: L-Amino acid deaminase, a novel stereoselective biocatalyst. Biotechnol. Adv. (2017) 35(6), 657-668.
Pollegioni L., Motta P., Molla G. L-amino acid oxidase as biocatalyst: a dream too far? Appl. Microbiol. Biotechnol. (2013) 97(21), 9323-9341.
Rosini E., Melis R., Molla G., Tessaro D, Pollegioni L. Deracemization and stereoinversion of α‐amino acids by l‐amino acid deaminase. Adv. Synth. Catal. (2017) 359(21), 3773-3781.
Soai K., Shibata T., Morioka H., Choji K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature (1995) 378, 767–768.
Wakayama M., Yoshimune K., Hirose Y., Moriguchi M. Production of D-amino acids by N-acyl-D-amino acid amidohydrolase and its structure and function. J. Mol. Catal. B Enzym. (2003) 23, 71–85
Author
Loredano Pollegioni, University of Insubria
