Analytical methods
Capillary Electrophoresis (CE)
Capillary electrophoresis (CE) is a separation technique that leverages the differences in ionic mobility of solvated ions in the presence of an applied electric field, where ionic mobility is dependent on the charge and stokes radius of the ions. CE has proven useful for analyzing a variety of biomolecules, ranging from single monosaccharides up to entire proteins. Relative to other separation techniques, such as liquid chromatography and gas chromatography, CE has a number of notable advantages: high efficiency, short separation times, and low sample volume requirements. Owing to the requirement for low sample volumes, CE has been used to characterize D-AAs in individual neurons.
In order to distinguish between the L- and D-forms of amino acids, chiral selectors are commonly employed. The differential interaction between the chiral forms of the analytes and the chiral selector result in their separation. Alternatively, CE without the use of such chiral selectors can also be used to separate and measure the amount of each enantiomer, provided they are first reacted with an optically pure chiral reagent (resulting in the formation of diastereomers prior to analysis).
CE can be paired with a variety of detection modalities for D-AA analysis, where laser-induced fluorescence (LIF) and mass spectrometry (MS) are two of the more common devices. Employing the aforementioned detection modalities provides sufficient sensitivity to enable the analysis of D-AAs in spite of limited sample volumes; these analyses can even be carried out at the subcellular level.
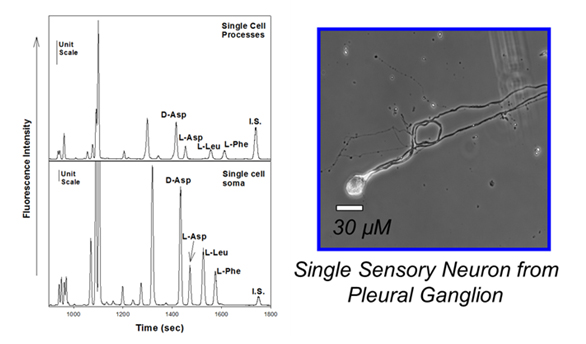
Electropherograms resulting from the subcellular analysis of the processes and cell soma of a single neuron from Aplysia.
Adapted from (Miao et al., J. Neurochem., 2006, 97(2), 595-606).
Liquid Chromatography (LC)
Since its advent, liquid chromatography (LC) has been the separation technique of choice for many analytical chemists. LC is well known as a robust approach for separating different chemical species on the basis of different retention times on a separation column as a consequence of differential interactions between the analytes, the liquid mobile phase, and the stationary phase. Much like the case described for CE, LC can discriminate on the basis of chirality by using a chiral stationary phase, incorporating additives which affect the retention time, or reacting the chiral species with an optically pure chiral reagent. Many of the early applications of LC for the analysis of D-AAs used fluorescence as its detection modality. This was done in part to take advantage of the sensitivity afforded by fluorescence detection, which was useful as these analyses were often targeting low-abundance analytes. The use of fluorescence detection was also employed because the precolumn derivatization reaction generated diasteroisomers. There are many examples of mass spectrometry being used as the detection modality for LC because of its sensitivity and selectivity.
References
Mothet J.P., Billard J.M., Pollegioni L., Coyle J.T., Sweedler J.V., Investigating brain D-serine: Advocacy for good practices. Acta Physiol., 2019, 16:e13257. doi: 10.1111/apha.13257.
Capillary electrophoresis (CE):
Miao H., Rubakhin S.S., Scanlan C.R., Wang L., Sweedler J.V., D-Aspartate as a putative cell-cell signaling molecule in the Aplysia californica central nervous system, J. Neurochem., 2006, 97(2), 595-606
Ota N., Rubakhin S.S., Sweedler J.V., D-Alanine in the islets of Langerhans of rat pancreas, Biochim. Biophys. Res. Comm., 2014, 447, 328–333
Otsuka K., Karuhaka K., Higashimori M., Terabe S. Optical resolution of amino acid derivatives by micellar electrokinetic chromatography with N-dodecanoyl-L-serine. J. Chromatogr., 1994, 680, 317–20
Scanlan C., Shi T., Hatcher N., Rubakhin S.S., Sweedler J.V., Synthesis, accumulation, and release of D-aspartate in the Aplysia californica central nervous system, J. Neurochem., 2010, 115(5), 1234–1244
Liquid chromatography (LC):
Dunlop D.S., Neidle A., The separation of D/L amino acid pairs by high-performance liquid chromatography after precolumn derivatization with optically active naphthylethyl isocyanate. Anal. Biochem., 1987, 165(1), 38-44
Dunlop D.S., Neidle A., McHale D., Dunlop D.M., Lajtha A., The presence of free D-aspartic acid in rodents and man, Biochim. Biophys. Res. Comm., 1986, 141(1), 27-32
Kimura T., Hamase K., Miyoshi Y., Yamamoto R., Yasuda K., Mita M., Rakugi H., Hayashi T., Isaka Y., Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Sci. Reports, 2016, 6: 26137
Author
Loredano Pollegioni, Università degli studi dell’Insubria
